mitotic drug treatment in cell culture Biology Diagrams The latter is accomplished by means of survivin, which is crucial in spindle assembly formation, and cyclin-dependent kinase inhibitor 1A (P21CIP1) that inhibits cell cycle progression at G 1 . Augmentation of HER2 occurs in 20-25 % of breast cancer types, and HER2-targeted therapy (trastuzumab and lapatinib) has been reported to increase Mitotic inhibitors bind to tubulin and inhibit its polymerization into microtubules. Microtubules are structures responsible for pulling the cell apart when it divides. Mitotic inhibitors affect cancer cells more than normal cells because cancer cells divide (mitotic cell division) more rapidly therefore are more susceptible to mitotic inhibition. Highly selective inhibitors developed against these targets showed robust cytotoxic activity in preclinical models. Similar to MT poisons, these anti-mitotics cause drug-specific and dose-dependent mitotic phenotypes through disruption of mitotic spindle morphology and MT-chromosome attachment or impairment of SAC (figure, bottom).

Functional redundancy between mitotic kinesins could be amongst the reasons that challenge the clinical efficacy of Eg5 inhibitors [59]. Interestingly, these Eg5 inhibitors induce cell death in combination with taxol even in taxol-resistant cancer cells, highlighting the therapeutic potential of Eg5 inhibition in combination with other Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res 2010; 70 : 10255-10264. Article CAS PubMed Google Scholar
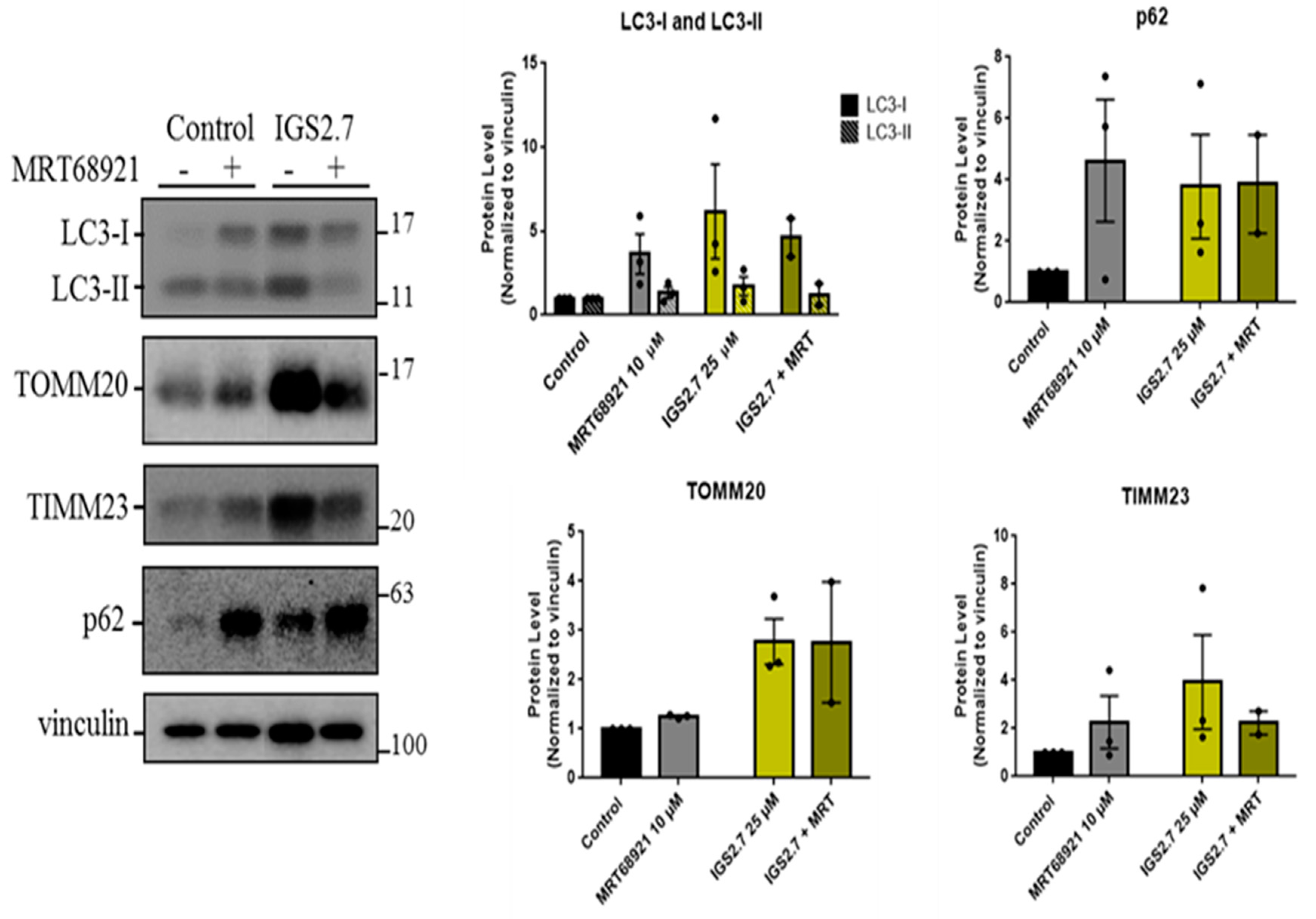
Antimitotic drugs in the treatment of cancer Biology Diagrams
This review aims to outline mitotic kinase inhibitors' roles as potential therapeutic targets and assess their suitability as a stand-alone clinical therapy or in combination with standard treatments for advanced-stage solid tumors, including triple-negative breast cancer (TNBC). Breast cancer poses a significant global health risk, with TNBC standing out as the most aggressive subtype Unfortunately, these so-called second-generation of antimitotics, encompassing mitotic blockers and mitotic drivers, have failed in clinical trials. Our recent understanding regarding the mechanisms of cell death during a mitotic arrest pointed out apoptosis as the main variable, providing an opportunity to control the cell fates and influence

The development of mitotic kinase inhibitors came as an outgrowth of studies on the molecular mechanisms of the cell cycle in the 1990s [38-40], which promised the development of precise inhibitors of mitosis without the toxic sequelae of classical chemotherapeutics. Yet for all the initial excitement, mitotic kinase inhibitors have now been The regulation of mitotic slippage in cells treated with microtubule/spindle poisons. The top panel shows a cell undergoing normal mitosis. Both APC/C and CRL2 ZYG11A/B ubiquitin ligases target the degradation of the mitotic regulator cyclin B1 to allow cells to progress from metaphase to anaphase and undergo mitotic exit. The middle panel shows a cell treated with an antimicrotubule drug that